The multiplex immunohistochemistry (mIHC) platform provides an automated, tissue sparing and
cost- effective solution for multiplex analysis of up 23 immune markers on a single FFPE slide. mIHC
analysis allows for thorough investigation of in situ immune contexture of tumor and stroma.
The multiplex approach allows for prospective and retrospective monitoring of clinical tissue samples:
- Baseline, in response to standard of care treatment, in response to immune treatment and longitudinal biopsies
- Primary (early and late stage) and metastatic disease
- Predictive biomarkers for treatment stratification
- Identification of response vs resistance to treatment
- On treatment changes in response to treatment
Methodology:
mIHC utilizes chromogen based immuno-detection and antibody stripping chemistry. mIHC antibody panels have been validated using single staining comparisons to multiplex staining on positive control tissues. Automated staining reduces batch variability and inclusion of positive control tissues in every staining batch assure accuracy. This technique offers deep, rapid and automated immune auditing
emphasizing spatial information. Panels are validated in brain, lung, liver, skin, pancreas, breast, lymph node, head and neck, and bone marrow tissue.
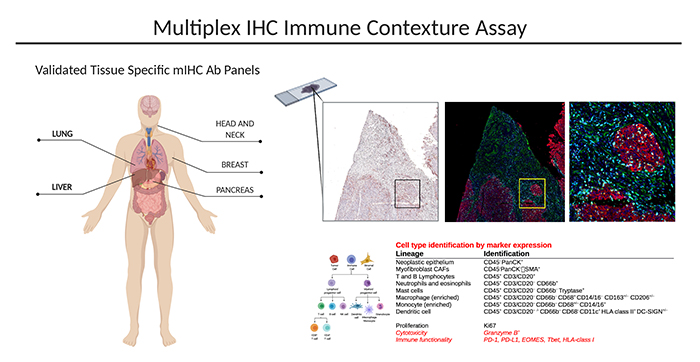
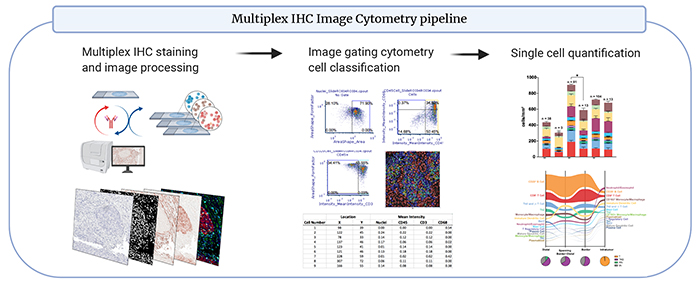
Discovery 23 panel:
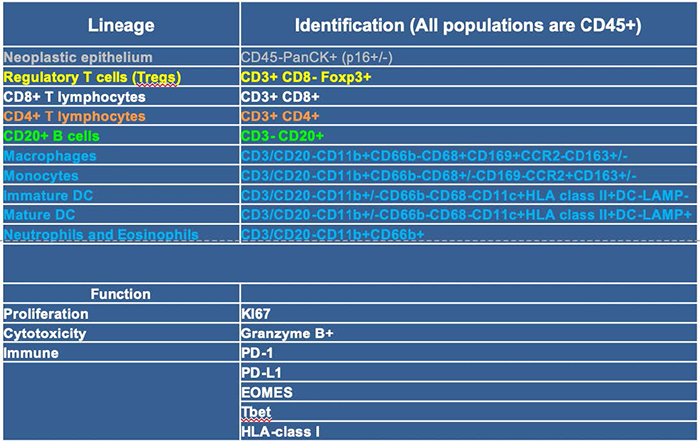
Discovery 23 panel results help guide subsequent deeper analysis with additional mIHC panels:
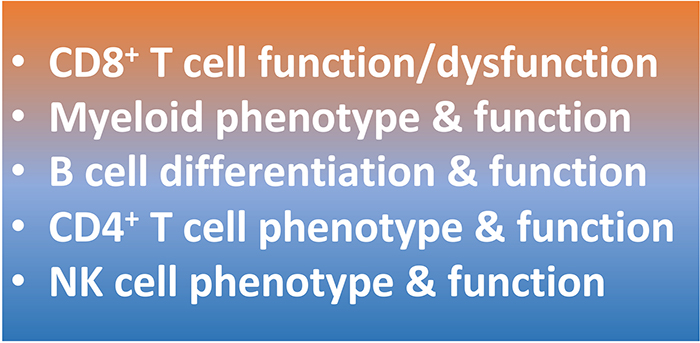
CUSTOM PANELS AND TISSUE SPECIFIC VALIDATION ARE AVAILABLE.
Specimen Requirements:
mIHC analysis is compatible with FFPE tissue or 20 gage needle core biopsy
See Our List of Available panels
Multiplex IHC Data Visualization and Interpretation Examples
Custom Panels Available Upon Request: IMCO@ohsu.edu