Mass cytometry by the time-of-flight or CyTOF is a single-cell proteomics technology capable of interrogation of more than 40 markers simultaneously in a liquid suspension. This technology empowers researchers to look deeper, unveiling new cell types and their functions.
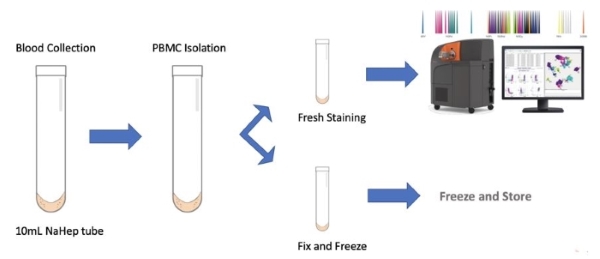
This high-resolution approach allows for prospective and retrospective, highly standardized and reproducible analysis of clinical samples (peripheral blood or tissue suspension):
- Immune cell activation and inhibition state
- Intra- and interpatient analyses
- Predictive biomarkers for treatment stratification
- Identification of response vs resistance to treatment
- On treatment changes reflective of therapeutic engagement
Methodology:
Helios™, a high-performance mass cytometer, is a state of-the-art instrument representing the greatest advances in mass cytometry and is designed to provide a new tool for bioanalytical single-cell detection and analysis. The Helios system analyzes individual cells labeled with stable heavy metal isotopes with negligible overlap which allows for unparalleled ability to generate high-resolution phenotypic and functional profiles of cells from normal and diseased states. Our CyTOF panels utilize the MaxPar Direct Assay which is designed and optimized for deep profiling of human blood. The use of the core assay with customizable additions is easily scalable, highly reproducible and validated across multiple patient cohorts and disease states.
Available panels:
- MaxPar: 30 markers allow for single cell resolution of 37 cimmunophenotypes
- MaxPar Plus: custom panel incorporates functional markers (activation, proliferation, check points) on the MaxPar backbone.
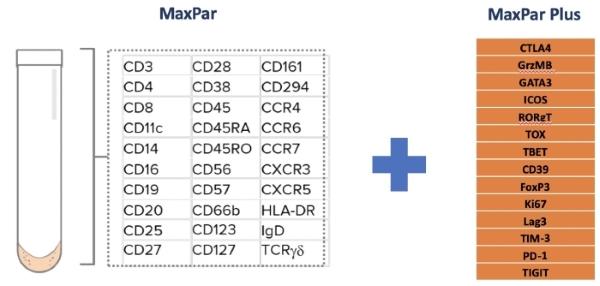
*CUSTOM PANELS and VALIDATION AVAILABLE
Specimen Requirements:
The CyTOF analysis is compatible with whole blood samples collected in NaHep tubes or processed frozen PBMCs (peripheral blood mononuclear cells).
Data visualization and interpretation examples
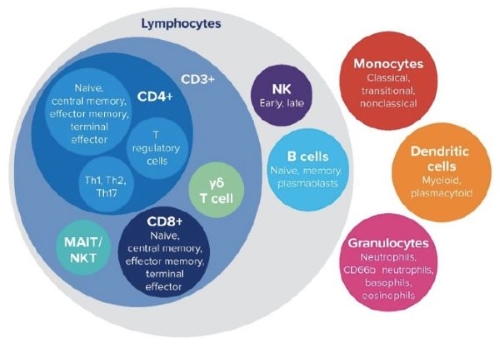
1. Immunophenotyping Analysis
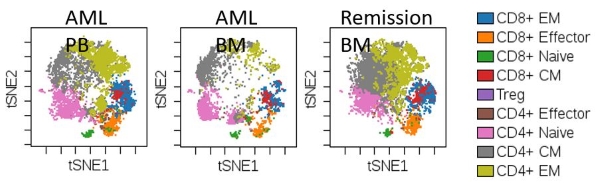
2. T-SNE Plots Analysis
3. Heat Map/Intensity Analysis
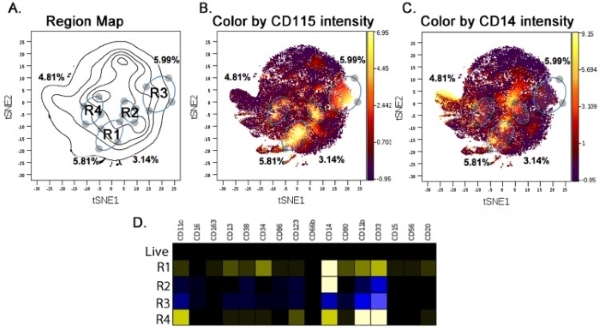
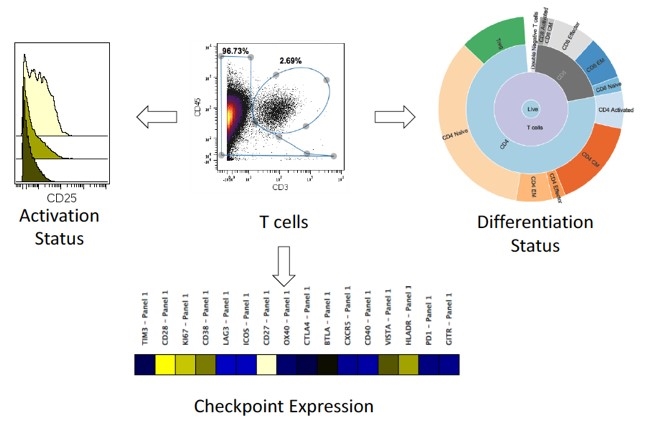
4. Combination Analysis
Publication List:
1. Reversible suppression of T cell function in the bone marrow microenvironment of acute myeloid leukemia. Lamble AJ, Kosaka Y, Laderas T, Maffit A, Kaempf A, Brady LK, Wang W, Long N, Saultz JN, Mori M, Soong D,LeFave CV, Huang F, Adams H 3rd, Loriaux MM, Tognon CE, Lo P, Tyner JW, Fan G, McWeeney SK, Druker BJ, Lind EF. Proc Natl Acad Sci U S A. 2020 Jun 23;117(25):14331-14341. doi: 10.1073/pnas.1916206117. Epub 2020 Jun 8. PMID: 32513686
2. CSF1R inhibitors exhibit antitumor activity in acute myeloid leukemia by blocking paracrine signals from support cells. Edwards DK 5th, Watanabe-Smith K, Rofelty A, Damnernsawad A, Laderas T, Lamble A, Lind EF, Kaempf A, Mori M, Rosenberg M, d'Almeida A, Long N, Agarwal A, Sweeney DT, Loriaux M, McWeeney SK, Tyner JW.Blood. 2019 Feb 7;133(6):588-599. doi: 10.1182/blood-2018-03-838946. Epub 2018 Nov 13. PMID: 30425048
3. Integrated functional and mass spectrometry-based flow cytometric phenotyping to describe the immune microenvironment in acute myeloid leukemia. Lamble AJ, Dietz M, Laderas T, McWeeney S, Lind EF. J Immunol Methods. 2018 Feb; 453:44-52. doi: 10.1016/j.jim.2017.11.010. Epub 2017 Nov 23. PMID: 29175391